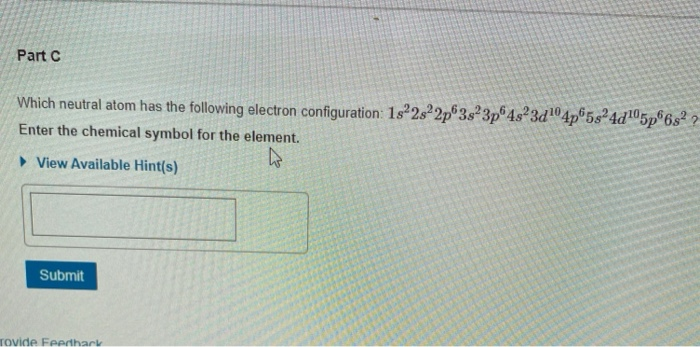
See the answer See the answer done loading. Part C which neutral atom has the following electron configuration.

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p2 Youtube
Identify the element that corresponds to the following electron configuration.

. This means that the atom youre looking for has an atomic mass of 23 which corresponds to the atomic mass of Vanadium a transition metal located. Which of the following does X represent. How many electrons are present in an atom of calcium that has the electron configuration 1s22s22p63s23p64s2.
First of all the number preceding the name of an electronic sub-level s p d is the principal quantum number n. What is the chemical symbol for the ion. Kr 5s2 4d10 5p4.
Which element has the following ground state configuration. There are 16 electrons and thus Z the nuclear charge is necessarily 16. Who are the experts.
The noble gas shorthand configuration is Ar4s1. 1 Answer anor277 Apr 16 2016. The atom have 3 valence electrons in its valence shell.
Which neutral atom has the following electron configuration. Urged to explain why an atom has only fixed energy levels Louis de Broglie postulated that if energy is particle-like perhaps matter is wave-like. The volume of an atom is mostly empty space b.
Which neutral atom has the following electron configuration. If Z 16 the element clearly silicon Si. What element is represented by the following electron configuration for its neutral atom.
Number of e 2 2 6 2 6 2 3 23. Use the periodic table to identify the element with the following electron configuration. Ca has 20 so 1s22s22p63s23p64s2 so it has 2 He is 1s2.
How many electrons does the ion have. N 4 This means that our mystery element exists in the fourth period of the periodic table. Neutral potassium atoms contain more neutrons than protons.
Experts are tested by Chegg as specialists in their subject area. Enter the chemical symbol for the element. Mendeleevs arrangement of elements in the periodic table was based on the observations and experiments of his predecessors who studied the mass and properties of elements.
This problem has been solved. To figure this out the element with the electron config of we first count the electrons. Electron configuration of iodine is 1s22s22p63s23p64s2 3d104p65s24d105p5 How many electrons are in the outer shell of the Ca and the He atoms.
This problem has been solved. The partial electron configuration of an atom with 12 electrons is shown1s22s22p6X. 1s22s22p63s23p64s2 In the box below provide the principle energy level of the valence electrons.
Chemistry Electron Configuration Electron Configuration. Calcium has the electron configuration 1s2 2s2 2p6 3s2 3p6 4s2 atomic number 20 H 1s1 Li 2s1 Na 3s1 K 4s1 so Ca is 4s2. A neutral atom has the following electron configuration.
Which neutral atom has the following electron configuration. Remember that for neutral atoms the number of electrons must equal the atomic number. Those are the small number placed at the top after the letters.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2. We review their content and use your feedback to keep the quality high. Enter the chemical symbol for the element.
Those are the small number placed at the top after the letters. The electron configuration for a ground-state potassium atom is 1s22s22p63s23p64s1. 1 2 and 3 so the valence energy level or Shell is three.
Adding up all the electrons from the given configuration will give. Electronic Configuration of give atom is 1s² 2s² 2p⁶ 3s² 3p¹ As the this atom has three energy levels ie. Answer 1 of 2.
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d9. The neutral atom contains as many electrons negatively charged particles as positively charged particles which reside in the nucleus. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2.
The highest present in this configuration is 4. Use the electron configuration shown below to answer the following question. In a ground-state hydrogen atom in which orbital is the electron.
Silver AG Aluminum has a total of _____ valence electrons. To figure this out the element with the electron config of we first count the electrons. Who are the experts.

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p5 Youtube

Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s1 Youtube
Solved Part C Which Neutral Atom Has The Following Electron Chegg Com
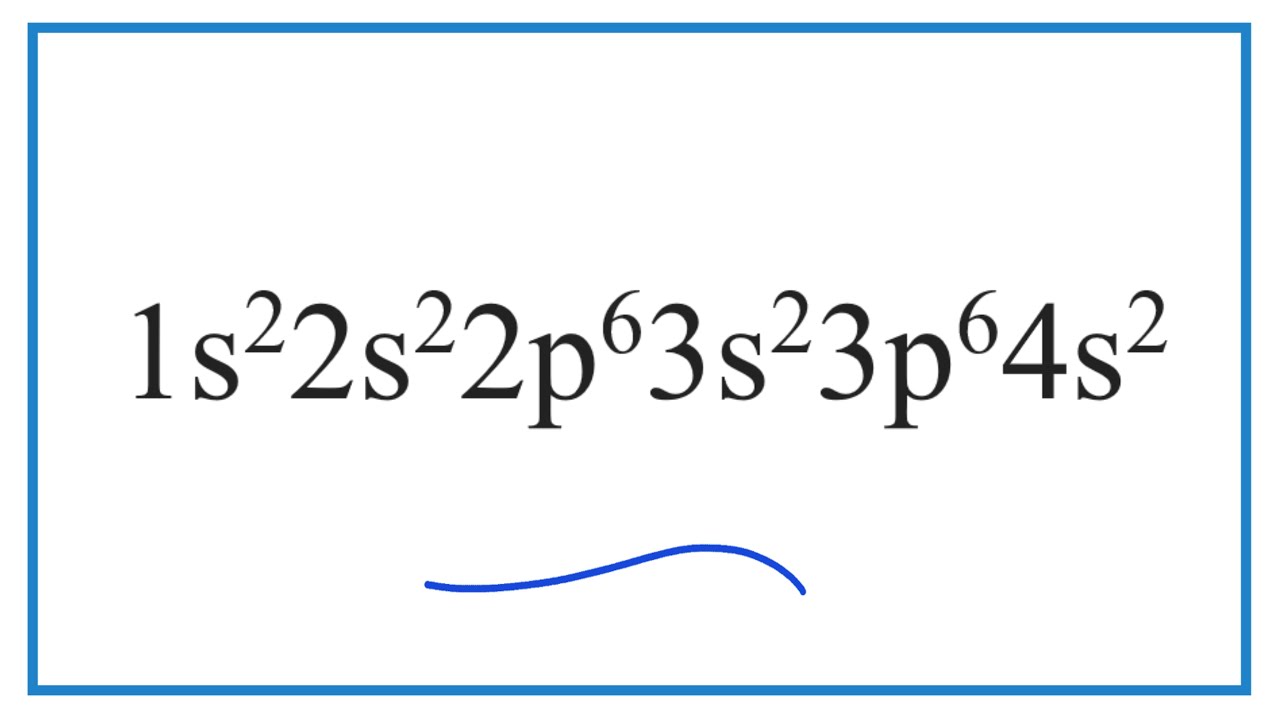
Which Element Has The Electron Configuration Of 1s2 2s2 2p6 3s2 3p6 4s2 Youtube
0 Comments